November 16, 2015
Circadian Clock Controls Insulin and Blood Sugar in Pancreas
Map of thousands of genes suggests new therapeutic targets for diabetes
The circadian clock is a molecular mechanism that regulates physiological responses of an organism to changes in the daily light cycle. Disruptions to the circadian clock have been linked to metabolic disorders such as cardiovascular disease, obesity, and type 2 diabetes. However, the exact mechanism underlying these disorders is poorly understood. Joseph Bass, a member of a CBC 2007 Catalyst team, is senior author on a recent Science paper, “Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion,” which provides new insights into how circadian regulatory processes impact pancreatic function. The work is a collaborative effort including several researchers from NU and UChicago.
CHICAGO — A new Northwestern Medicine study has pinpointed thousands of genetic pathways an internal body clock takes to dictate how and when our pancreas must produce insulin and control blood sugar, findings that could eventually lead to new therapies for children and adults with diabetes.
The body’s circadian clocks coordinate behaviors like eating and sleeping, as well as physiological activity like metabolism, with the Earth’s 24-hour light-dark cycle. There’s a master clock in the brain, as well as peripheral clocks located in individual organs. When genetics, environment or behavior disrupt the synchrony of these clocks, metabolic disorders can develop.
 In a previous publication in Nature, Northwestern Medicine investigators showed that a circadian clock in the pancreas is essential for regulating insulin secretion and balancing blood sugar levels in mice. The scientists demonstrated that knocking out clock genes led to obesity and type 2 diabetes, but they still had much to learn if they wanted to manipulate clock action to treat the conditions.
In a previous publication in Nature, Northwestern Medicine investigators showed that a circadian clock in the pancreas is essential for regulating insulin secretion and balancing blood sugar levels in mice. The scientists demonstrated that knocking out clock genes led to obesity and type 2 diabetes, but they still had much to learn if they wanted to manipulate clock action to treat the conditions.
“We knew that the pancreas didn’t work if we removed these clock genes, but we didn’t know how the genes were affecting the normal function of the pancreas,” said principal investigator Dr. Joe Bass (right), chief of endocrinology at Northwestern University Feinberg School of Medicine and a Northwestern Medicine physician.
Clock genes are responsible for producing transcription factors, special proteins that help tell a cell how to function.
In the new study, published Nov. 6 in Science, Bass’s laboratory revealed thousands of genes in the pancreas that the clock’s transcription factors control in rhythm with the planet’s daily rotation from light to dark.
“We established a new gene map that shows how the entire repertoire of factors produced in the pancreas maintain and anticipate daily changes in the external environment,” Bass said. “These factors are all tied to the rotation of the Earth — to the timekeeping mechanism that has evolved to control when we sleep, wake up, eat and store nutrients each day.”
Bass’s team focused on cells in the pancreas called beta cells, which secrete insulin into the blood stream to help the body absorb glucose — sugar — to use for energy. Using genome-wide sequencing technology on beta cells with both intact and disrupted clock gene function, the scientists were able to lay out the map of transcription factors and genes.
In ongoing research, Bass’s group continues to study how the body’s circadian clocks interact and how their rhythm is thrown off — not just in diabetes, but also during the normal aging process and from day-to-day conditions like jetlag, stress or dietary changes.
“This study reinforces the idea that clocks operating in cells are fundamental to health,” Bass said. “They represent an important untapped target for improving the functions of cells in the pancreas.”
Bass is also the Charles F. Kettering Professorship of Medicine at Feinberg. Other Northwestern authors include Dr. Grant Barish, Mark Perelis, Biliana Marcheva, Kathryn Ramsey, Clara Bien Peek, Hee-kyung Hong, Matthew Schipma, Dr. Akihiko Taguchi, Dr. Wenyu Huang, Chiaki Omura and Amanda Allred.
This study was supported by National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK090625, NIH National Institute on Aging grant P01AG011412, the Chicago Biomedical Consortium S-007, Juvenile Diabetes Research Foundation grants 17-2013-511, 1-INO-2014-178-A-V and 1-INO-2015-23-A-V, University of Chicago Diabetes Research and Training Center grant P60DK020595; NIDDK T32 grant DK007169; National Heart, Lung, and Blood Institute T32 grant HL007909 and Defense Advanced Research Projects Agency grant D12AP00023.
Article adapted from: NU News, story by Nora Dunne, a content specialist at the Northwestern Feinberg School of Medicine
Publication attributed to the CBC Catalyst Award: Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015 Nov 6;350(6261):aac4250. (PubMed)
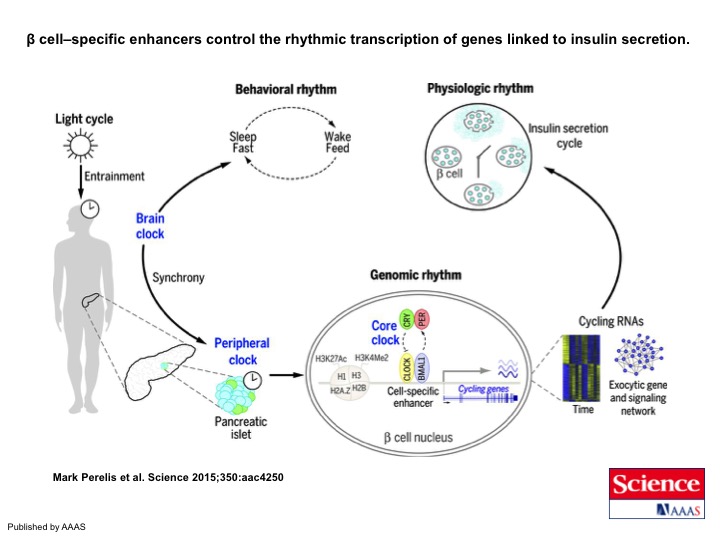
Graphic abstract: β cell–specific enhancers control the rhythmic transcription of genes linked to insulin secretion. Peripheral clocks maintain glucose homeostasis across the sleep-wake cycle by gating β cell insulin secretion through genome-wide transcriptional control of the assembly and trafficking of insulin secretory vesicles. Clock transcription factors bind within cell type–specific enhancer neighborhoods of cycling genes, revealing the mechanisms that synchronize rhythmic metabolism at transcriptional and physiologic levels across the light-dark cycle. Perelis et al. Science. 2015 Nov 6;350(6261):aac4250. (PubMed)
CBC Catalyst Award (2007): Joseph Bass (NU), Graeme I. Bell (UChicago) and Nissim Hay (UIC) for project: Chicago Consortium in Diabetes and Obesity Genetics
