Poster # 7
IMPARTING HORMONE IMPRINT ON ESTROGEN RESPONSIVE GENES LEADING TO INCREASED RISK OF UTERINE FIBROIDS BY DEVELOPMENTAL EXPOSURE TO ENDOCRINE DISRUPTING CHEMICALS
Authors: Qiwei Yang, University of Chicago, Chicago, IL, USA; Mohamed Ali, Ain Shams University, Cairo, Egypt; Ayman Al-Hendy, University of Chicago, Chicago, IL, USA
PRESENTER’S INFO:
Name: Qiwei Yang
Email: yangq@bsd.uchicago.edu
Title: Faculty
Affiliation: The University of Chicago
Department: Obstetrics and Gynecology
Advisor: Ayman Al-Hendy
Advisor’s Email: aalhendy@bsd.uchicago.edu
Abstract:
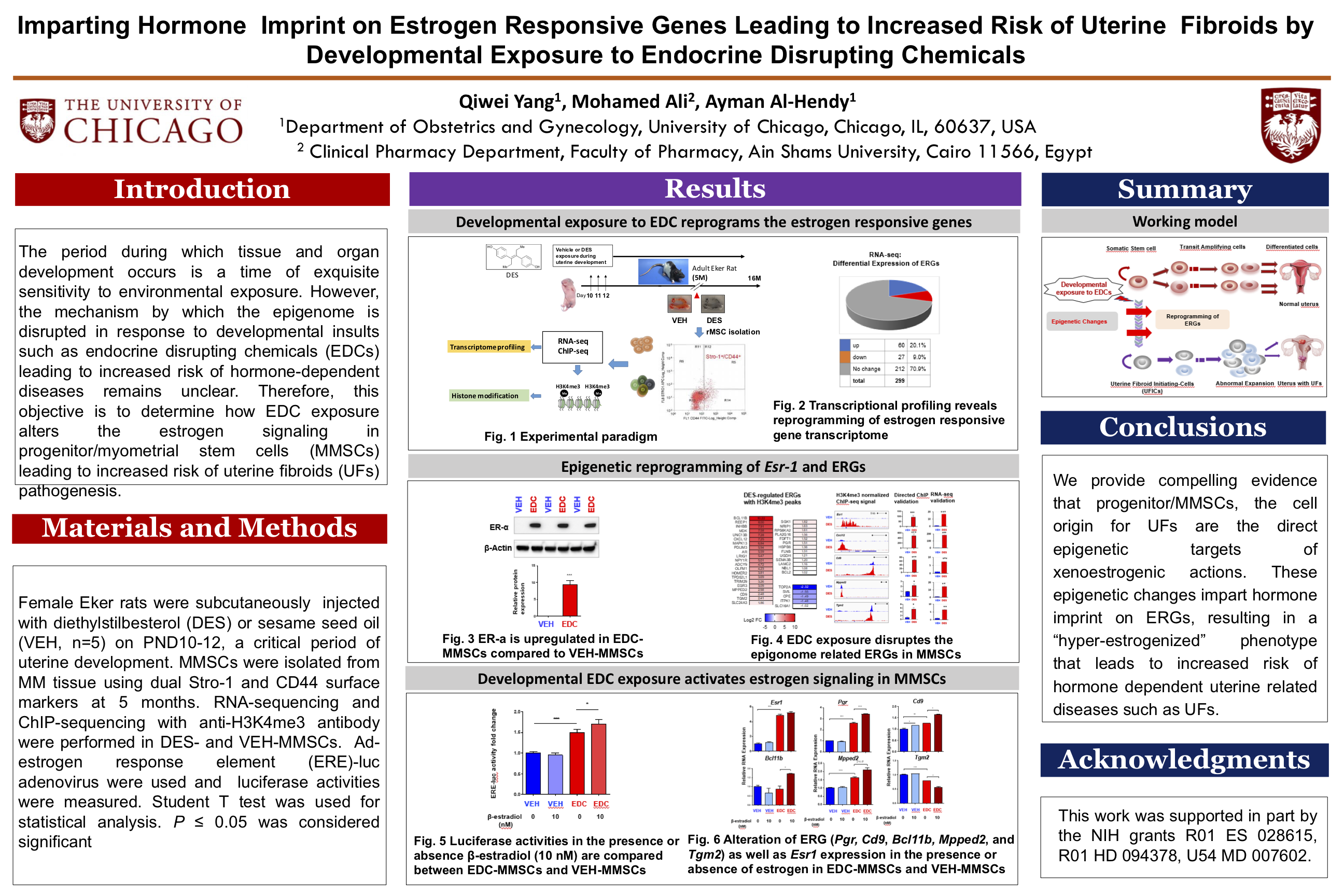
Introduction: The period during which tissue and organ development occurs is a time of exquisite sensitivity to environmental exposure. However, the mechanism by which the epigenome is disrupted in response to developmental insults such as endocrine disrupting chemicals (EDCs) leading to increased risk of hormone-dependent diseases remains unclear. Therefore, this objective is to determine how EDC exposure alters the estrogen signaling in progenitor/myometrial stem cells (MMSCs) leading to increased risk of uterine fibroids (UFs) pathogenesis.
Materials and Methods: Female Eker rats were subcutaneously injected with diethylstilbesterol (DES) or sesame seed oil (VEH, n=5) on PND10-12, a critical period of uterine development. MMSCs were isolated from MM tissue using dual Stro-1 and CD44 surface markers at 5 months. RNA-sequencing and ChIP-sequencing with H3K4me3 antibody were performed in DES- and VEH-MMSCs. Ad-estrogen response element (ERE)-luc adenovirus were used and luciferase activities were measured. Student T test was used for statistical analysis. P ≤ 0.05 was considered significant.
Results: Our previous genome-wide transcriptome and ChIP-seq analysis showed that the increased expression of estrogen responsive genes (ERGs) was correlated with the H3K4me3 mark (p<0.05). Importantly, the master regulator (estrogen receptor) for estrogen signaling was H3H4me3-reprogammed in EDC-MMSCs. Furthermore, ERE-related luciferase activity was significantly higher in EDC-MMSCs compared to VEH-MMSCs. Notably, estradiol treatment further increased the luciferase activity in EDC-MMSCs but not in VEH-MMSCs. Estradiol treatment significantly increased the expression of reprogrammed ERGs including Pgr, Cd9, Bcl11b, and Mpped2, while the expression of Pgr, Bcl11b, and Mpped2 showed no changes in VEH-MMSCs after treatment with estradiol.
Conclusions: We provide compelling evidence that progenitor/MMSCs, the cell origin for UFs are the direct epigenetic targets of xenoestrogenic actions. These epigenetic changes impart hormone imprint on ERGs, resulting in a “hyper-estrogenized” phenotype that leads to increased risk of hormone dependent uterine related diseases such as UFs.
Poster: To download / open the poster as a PDF file in a new window click on the image below.
No Fields Found.