March 4, 2020 I Jola Glotzer
Atomic structure of Paramyxoviruses
Global Biodefense features the CBC Catalyst Award-supported work of to Yuan He, NU on a paramyxovirus 3D structure
Infective powers of viruses are a topic of ongoing daily conversations and worries around the world in the era of the present-day coronavirus epidemics. Hence, a recent paper in PNAS, titled “Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation,” published by a team of NU scientists led by Drs. Lamb and He is a very timely one, as it provides answers to some key questions about the virus replication mechanisms.
Using a relatively novel technique, cryogenic electron microscopy (cryo-EM), the NU scientists were able to reconstruct a 3D structure of a multi-protein complex of paramyxovirus RNA polymerase. Examples of paramyxoviruses that cause human diseases include parainfluenza, mumps and respiratory syncytial virus (RSV). The newly published data revealed two surprising findings. First, the polymerase complex, which consists of five proteins, contains two proteins which are “brand new” and have not been identified before. Second, the polymerase plays a dual role switching between the RNA and DNA polymerizing activity during the processes of transcription and genome replication, respectively.
Although the paramyxoviruses constitute a different family than coronaviruses, both families are enveloped, RNA-type viruses, sharing many similarities between their replication and infection cycles. Thus, the pioneering work of Drs. He and Lamb, brings the scientific community one step closer to understanding the particularities of the virus replication machinery critical for its survival. The approach employed in the paper to decipher paramyxovirus polymerase–the cryo-EM–could become a powerful tool to study other viruses and critical for their survival proteins. This could facilitate and speed-up more accurate modeling of targeted therapeutic approaches, including the development of effective vaccines.
Congratulations to He, Lamb and both of their teams on this important work and discovery!
A CBC Catalyst Award that Dr. He received in 2015 is acknowledged for partially funding the published research. Dr. Lamb is a CBC affiliate as well–he served on the CBC Recruitment Resources Fund Board (2006-2011) and was a member of the CBC Spark Council (2008-2011). The CBC would like to take this opportunity to thank Dr. Lamb for his support of the CBC programming in the past.
Enjoy the article reposted below from the Global Biodefence.
February 17, 2020 | Global Biodefense Staff | Global Biodefense
Atomic Structures Mapped for First Time in Paramyxoviruses
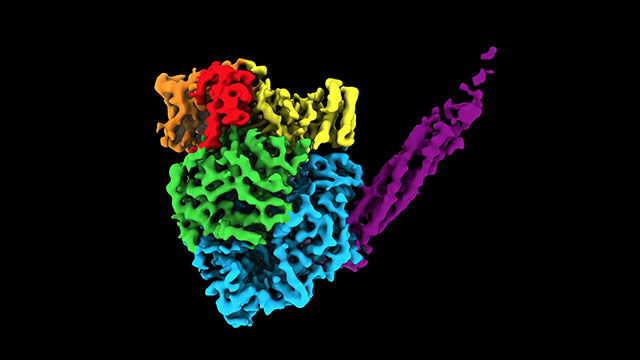
The atomic structure of a polymerase in human parainfluenza virus 5 reveals an irregular, round-shaped globule with a long tail made of four phosphoproteins. The structure contains more than 2,000 amino acids and five proteins. Credit: Northwestern University. Source: Global Biodefence.
Northwestern University researchers have, for the first time, determined the 3D atomic structure of a key complex in paramyxoviruses, a family of viruses that includes measles, mumps, human parainfluenza and respiratory syncytial virus (RSV).
This information could help others design and develop antiviral drugs for these viruses as well as for coronavirus, which functions similarly to paramyxoviruses.
“This takes some of the guesswork out of designing drugs,” said Northwestern’s Robert Lamb, who co-led the study. “Traditionally, you have to develop drugs randomly and hope you hit a target, but it doesn’t happen very often.”
To find the unique structure, researchers used cryogenic electron microscopy (cryo-EM). The relatively new technique enables researchers to peer inside molecules to determine the 3D shape of proteins, which are often thousands of times smaller than the width of a human hair. Before cryo-EM, researchers mainly used X-ray crystallography, which is incapable of capturing high-resolution images of this enzyme. Called a polymerase, the enzyme assembles RNA molecules.
“Crystallography only works for very orderly and organized proteins,” said Northwestern’s Yuan He, who co-led the study. “Virus polymerase complexes are too big to be crystallized and don’t have uniformity.”
The study will be published on Feb. 17 in the Proceedings of the National Academy of Sciences.
Lamb is the Kenneth F. Burgess Professor of Molecular Biosciences in Northwestern’s Weinberg College of Arts and Sciences and an investigator of the Howard Hughes Medical Institute. Yuan He is an assistant professor of molecular biosciences in Weinberg.
Although the first documented case of mumps occurred in the 5th century and measles in the 9th century, researchers did not have the equipment to characterize their atomic structures until relatively recently. A trio of biophysicists received the 2017 Nobel Prize in Chemistry for developing cryo-EM, which ultimately opened the door for Lamb and He.
Cryo-EM works by blasting a stream of electrons at a flash-frozen sample to take many 2D images. For this study, He and his team captured hundreds of thousands of images of one sample of human parainfluenza virus 5 polymerase. The team then used computational algorithms to reconstruct a 3D image.
The resulting image was an irregular, round-shaped globule with a long tail made of four phosphoproteins (or proteins containing phosphorous). The structure contains more than 2,000 amino acids and five proteins.
“Part of the image was expected,” Lamb said. “But part of it was a surprise. Two of the proteins are completely new. They have never been seen before.”
Another surprise: the team found that this virus uses the same protein to switch between genome replication and transcription.
“This machinery has a dual-function,” He said. “It gets both jobs done with one enzyme. The virus’s genome is so small, and this gives it economy of scale.”
Lamb and He hope this work can help others design and develop new drugs for illnesses such as measles and mumps, which have experienced outbreaks in past years.
“A lot of people don’t want to get vaccinated, and they’re getting the disease,” Lamb said. “And for people who are vaccinated, it still takes three to four weeks for that vaccine to take effect. We need more antiviral drugs so people who get infected can be treated immediately.”
The study, “Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation,” was supported by a Cornew Innovation Award from the Chemistry of Life Processes Institute at Northwestern University, the Chicago Biomedical Consortium, and the National Institutes of Health (award numbers R01-GM135651 and T32-GM008382).
Publication attributed to the *CBC funding:
Abdella R, Aggarwal M, Okura T, Lamb RA, He Y. Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc Natl Acad Sci U S A. 2020 Feb 19. [Epub ahead of print] (PubMed)
SOURCE:
Adapted (with modifications) from Global Biodefence, by Global Biodefence Staff, published on February 17, 2020.
Featured CBC Community members:
Yuan He, NU
- CBC Postdoctoral Research Award (2016):
▸ Proteomic and Structural Analysis of Human RNA Pol III Transcription Machinery
PIs: Yan Han (postdoc) and Yuan He (NU) - *CBC Catalyst Award (2015):
▸ Toward the Visualization of Spliceosomal Intermediates
PIs: Yuan He (NU), Joseph Piccirilli (UChicago) and Jonathan Staley (UChicago)
Robert Lamb, NU
- 13th CBC Annual Symposium (2015):
▸ The Unseen Majority: Microbes in Health and Disease
Robert Lamb (NU) – Symposium Speaker - CBC Spark Council (2008-2011):
Robert Lamb (NU) – Council Member - CBC Recruitment Resources Fund Board (2006-2011):
Robert Lamb (NU) – Board Member
ARTICLES PUBLISHED IN THE PAST ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
November 8, 2019
▸ Structure of chromatin remodeling complex SWI/SNF resolved
Two CBC Awards to Han Yan and Yuan He, NU, have supported research recently published a preprint in bioRxiv, describing the structure of SWI/SNF complex bound to a nucleosome at near atomic resolution