October 16, 2019 | Jola Glotzer
Dissecting SOD2 tumorigenic properties
In a recent PNAS paper, two CBC Awardees, David Gius, NU and Jonna Frasor, UIC explore the mechanisms of mitochondrial superoxide dismutase (SOD2) cancerogenic activity in late-stage breast cancer
Mitochondrial superoxide dismutase (SOD2) is a known suppressor of tumor initiation. However, in late-stage solid tumors, SOD2 becomes tumorigenic by promoting both cancer cells’ invasion and metastasis.
In a recent PNAS publication, titled “SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer”, the authors report that overexpression of SOD2 causes accumulation of its acetylated form (SOD2K68Ac), which in turn increases mitochondrial reactive oxygen species (mtROS) as well as hypoxia-induced factor 2α (HIF2α) activity. Being a potent promoter of cancer stem cell formation, HIF2α is thought to be responsible for breast cancer treatment failure and cancer metastatic recurrence. The authors demonstrate that, indeed, increased levels of SOD2 and HIF2α coexist and colocalize in metastatic lesions as compared with primary tumors from the same patients, suggesting that SOD2K68Ac accumulation observed in late-stage cancers may be the driver behind the observed increased tumor aggressiveness and poorer outcomes through a mechanism involving stimulation of cancer stem cell development.
Two CBC awardees contributed to the current study: David Gius, NU and Jonna Frasor, UIC. Gius, Professor of Radiation Oncology and Pharmacology at NU’s Feinberg School of Medicine, is second senior author on the current study. Frasor, Professor at the Department of Physiology & Biophysics, College of Medicine, UIC, is one of the co-authors on the PNAS paper. A CBC Catalyst Award that Gius received in 2015 for the project “Fluorescent Probes for Live-cell Imaging of Sirtuin Activity: Application to Breast Cancer” partially funded the publication.
Publication attributed to *CBC funding:
He C, Danes JM, Hart PC, Zhu Y, Huang Y, de Abreu AL, O’Brien J, Mathison AJ, Tang B, Frasor JM, Wakefield LM, Ganini D, Stauder E, Zielonka J, Gantner BN, Urrutia RA, *Gius D, Bonini MG. SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer. Proc Natl Acad Sci U S A. 2019 Oct 7. [Epub ahead of print] (PubMed)
ABSTRACT:
Mitochondrial superoxide dismutase (SOD2) suppresses tumor initiation but promotes invasion and dissemination of tumor cells at later stages of the disease. The mechanism of this functional switch remains poorly defined. Our results indicate that as SOD2 expression increases acetylation of lysine 68 ensues. Acetylated SOD2 promotes hypoxic signaling via increased mitochondrial reactive oxygen species (mtROS). mtROS, in turn, stabilize hypoxia-induced factor 2α (HIF2α), a transcription factor upstream of “stemness” genes such as Oct4, Sox2, and Nanog. In this sense, our findings indicate that SOD2K68Ac and mtROS are linked to stemness reprogramming in breast cancer cells via HIF2α signaling. Based on these findings we propose that, as tumors evolve, the accumulation of SOD2K68Ac turns on a mitochondrial pathway to stemness that depends on HIF2α and may be relevant for the progression of breast cancer toward poor outcomes.
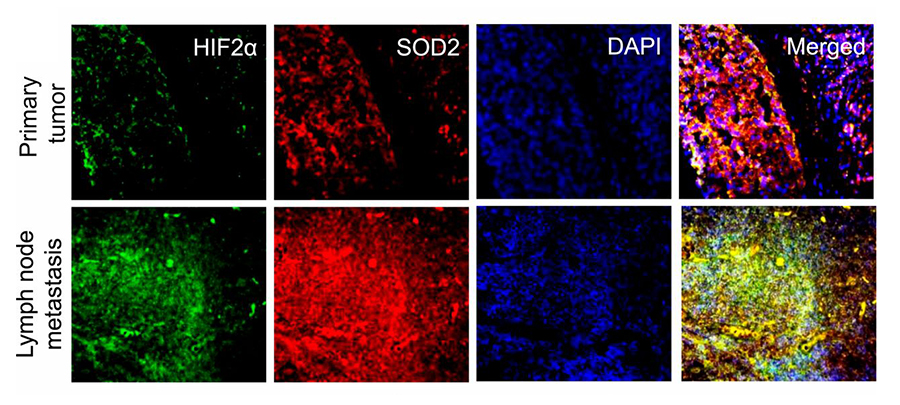
SOD2 and HIF2α are enriched in metastasis compared to primary tumors from the same patients. Micrographs of primary tumor and metastatic tumors stained for HIF2α (green) and SOD2 (red). (Source: PNAS)
ACKNOWLEDGMENTS:
We thank Drs. Kristine Ansenberger-Fricano, Micaela Vargas, and Kraig Thierault for preliminary studies done in the early phases of this project and Ms. Kelly Dille and Mr. Ram Patel for technical assistance. We acknowledge funding from US Department of Defense awards 67263-RT-REP and 911NF-07-R-0003-04 to M.G.B. and 5RO1HL125356, 7RO1AI131267, 1RO1CA216882, and 1R01ES028149 to M.G.B. D. Gius is supported by National Cancer Institute grants 2R01CA152601, 1R01CA152799, 1R01CA168292, and 1R01CA214025, the Avon Foundation for Breast Cancer Research, the Lynn Sage Cancer Research Foundation, the Zell Family Foundation, and the Chicago Biomedical Consortium, as well the Searle Funds at The Chicago Community Trust. We also acknowledge the contributions of the anonymous reviewers for their thorough review of this study and suggestions that much improved this study.
Featured CBC Community member(s):
David Gius, NU
- *CBC Catalyst Award (2015):
▸ Fluorescent Probes for Live-cell Imaging of Sirtuin Activity: Application to Breast Cancer
PIs: David Gius (NU) and Bryan Dickinson (UChicago)
Jonna Frasor, UIC
- CBC Catalyst Award (2014):
▸ Photoaffinity-based Protein Profiling Approach to Discover Estrogen Receptor/Coactivator Inhibitors
PIs: Jonna Frasor and Terry Moore (UIC) and Geoffrey Greene (UChicago) - CBC Catalyst Award (2009):
▸ A Systems Biology Understanding of Estrogen Receptor Action
PIs: Jonna Frasor and Yang Dai (UIC) and Serdar Bulun (NU)
ARTICLES PUBLISHED IN THE PAST ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
June 5, 2019
▸ Meet a double-agent, MnSOD
David Gius, NU, explains in a recent Nature Communications paper — partially funded by a CBC Catalyst Award — how MnSOD can act as a pro-, or an anti-cancer agent
February 5, 2019
▸ Macrophages – a dance of life with death
Five (!) CBC community members collaborate on how to speed up heart recovery after an infarct

