Poster # 5
ESTROGEN SIGNALING IN THE VENTROMEDIAL HYPOTHALAMUS MODULATES ADIPOSE TISSUE METABOLIC ADAPTATION
Co-authors: Valeria Torres Irizarry1, Hui Ye1, Pei Luo1, Leslie Carrillo-Sáenz1,2, Xiaohua Yang1, Nimisha Antony1, Yanlin He1,3, Yuwei Jiang2, Pingwen Xu1,2,*
1Division of Endocrinology, Diabetes and Metabolism, College of Medicine, The University of Illinois at Chicago, Chicago, Illinois
2Pennington Biomedical Research Center, Louisiana State University System
3Department of Physiology and Biophysics, The University of Illinois at Chicago
PRESENTER’S INFO:
Name: Valeria C Torres Irizarry
Email: vtorre26@uic.edu
Twitter Handle: @vtorre26
Title: Student
Affiliation: University of Illinois Chicago
Department: Division of Endocrinology, Diabetes and Metabolism
Advisor: Dr. Pingwen Xu
Advisor’s Email: pingwenx@uic.edu
Abstract:
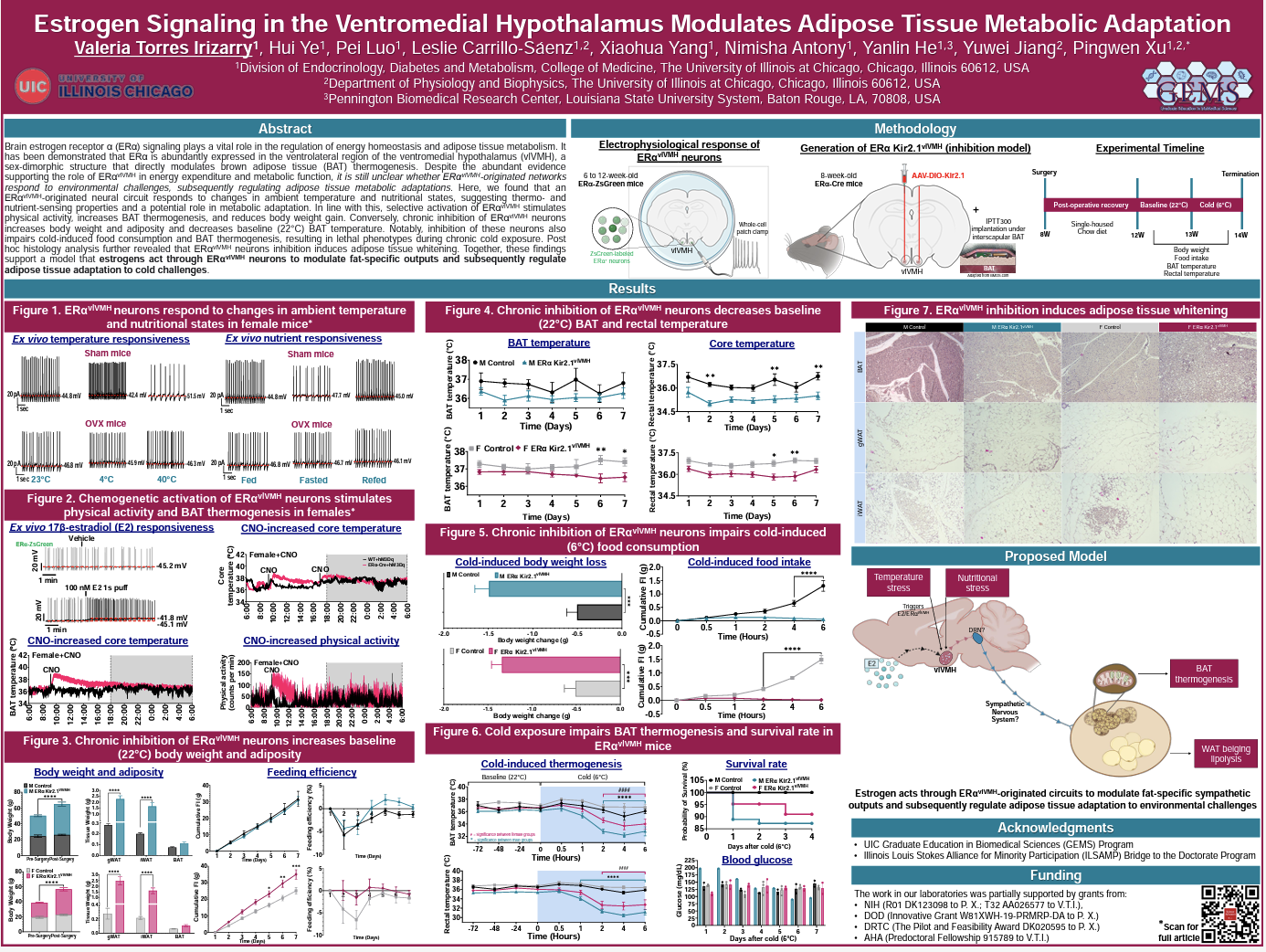
Brain estrogen receptor α (ERα) signaling plays a vital role in the regulation of energy homeostasis and adipose tissue metabolism. It has been demonstrated that ERα is abundantly expressed in the ventrolateral region of the ventromedial hypothalamus (vlVMH), a sex-dimorphic structure that directly modulates brown adipose tissue (BAT) thermogenesis. Despite the abundant evidence supporting the role of ERα-vlVMH in energy expenditure and metabolic function, it is still unclear whether ERα-vlVMH-originated networks respond to environmental challenges, subsequently regulating adipose tissue metabolic adaptations. Here, we found that an ERα-vlVMH-originated neural circuit responds to changes in ambient temperature and nutritional states, suggesting thermo- and nutrient-sensing properties and a potential role in metabolic adaptation. In line with this, selective activation of ERα-vlVMH stimulates physical activity, increases BAT thermogenesis, and reduces body weight gain. Conversely, chronic inhibition of ERα-vlVMH neurons increases body weight and adiposity and decreases baseline (22°C) BAT temperature. Notably, inhibition of these neurons also impairs cold-induced food consumption and BAT thermogenesis, resulting in lethal phenotypes during chronic cold exposure. Post hoc histology analysis further revealed that ERα-vlVMH neurons inhibition induces adipose tissue whitening. Together, these findings support a model that estrogens act through ERα-vlVMH neurons to modulate fat-specific outputs and subsequently regulate adipose tissue adaptation to cold challenges.
Poster: To download / open the poster as a PDF file in a new window click on the image below.
No Fields Found.