November 19, 2019 | Jola Glotzer
Problems with metal/organic interfaces…
Explained and solved by CBC Awardee Laura Sanchez, UIC, in a recent Chemical Communications publication
Congratulations to the Sanchez lab at UIC on a recent publication in the journal Chemical Communications, titled “Chemically transformed monolayers on acene thin films for improved metal/organic interfaces.”
The authors explore ways to improve organic semiconductors for optoelectronic and sensing applications by chemically modifying the semiconductor’s surface. They demonstrate how specific treatments of a highly reactive anhydride terminated self-assembled monolayer (SAM) on tetracene – a thin film on the organic semiconductor’s surface – significantly boost an ability to interact with metal films deposited on its top. The methodology described in the paper is expected to improve many other optoelectronic applications.
Laura Sanchez, PhD, who is senior author on the publication is Assistant Professor in the Department of Medicinal Chemistry and Pharmacognosy at UIC with a courtesy appointment in Chemistry.
The HTX TM-sprayer used in the experiments described in the paper — a sample preparation system designed to provide the highest quality matrix deposition on 2D biological samples — was funded by the CBC through a CBC Catalyst Award which Sanchez received in 2016.
Publication attributed to *CBC funding:
Li F, Hopwood JP, Galey MM, Sanchez LM, Ciszek JW. Chemically transformed monolayers on acene thin films for improved metal/organic interfaces. Chem Commun (Camb). 2019 Nov 5. [Epub ahead of print] (PubMed)
ABSTRACT:
Anhydride terminated acene thin films were chemically transformed to thiol or carboxylic acid functionalities, groups heretofore incompatible with monolayer reactions. The molecular surface imparts large rate acceleration when imides are formed, while disfavored disulfides can be formed from the thiols. The modified surface imparts improved adhesion to top metal contacts in flexible/bendable applications.
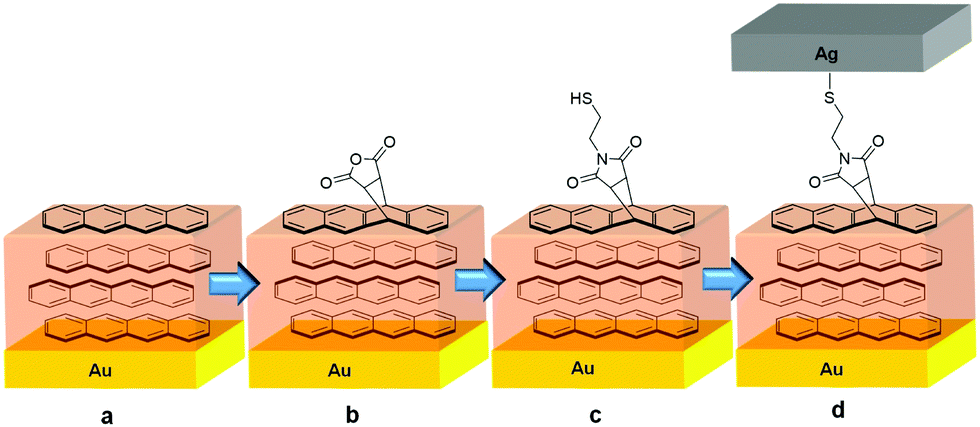
Functionalization of a tetracene film (a) via previously established Diels–Alder chemistry to form the precursor anhydride films (b). Transformation of SAMs provides further derivatization, in this case generating an imide (c), which allows for coordination of a thermally deposited metal film (d). (Source: Chem. Commun., 2019)
ACKNOWLEDGMENTS:
This work was funded by the National Science Foundation (NSF), award No. 1665433. This research utilized a scanning electron microscope, which was funded by the NSF Major Research Instrumentation (MRI) award No. 1726994. The MALDI-TOF MS used for this research was supported by UIC startup funds (LMS) and the HTX TM-sprayer was funded by the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (LMS). JPH would like to thank the Arthur J. Schmitt Foundation for additional funding.
Featured CBC Community member(s):
Laura Sanchez, UIC
- *CBC Catalyst Award (2016):
▸ Capturing Host-Microbiome Chemical Communication
PIs: Laura Sanchez (UIC) and Mark Mandel (NU)
ARTICLES PUBLISHED IN THE PAST ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
April 25, 2019
▸ Understanding cell-to-cell communication in cancer
Two CBC awardees, Laura Sanchez and Joanna Burdette, UIC, developed a novel method of sample preparation to detect small molecule exchange using imaging mass spectrometry
October 17, 2018
▸ Building a library of… bacteria
Two CBC awardees, Laura Sanchez and Brian Murphy, UIC, co-PIs on a $1.7 million NIH grant to create a reference library of bacteria
October 12, 2018
▸ Ovarian or fallopian tube cancer?
Two UIC scientists with links to CBC, Joanna Burdette and Laura Sanchez, collaborate to decipher the origin and the mechanism of spread of ovarian cancer