November 19, 2019 | Jola Glotzer
Modeling MEK4 Kinase Inhibitors
Four past CBC Awardees working at the CBC Lever Award-supported NU Medicinal and Synthetic Chemistry Core (ChemCore) at the Center for Molecular Innovation and Drug Discovery (CMIDD) contribute
MEK4 is a kinase that is a part of a second tier signaling protein of the canonical three-tier MAPK cascade implicated to play a carcinogenic role in several cancer types. Specifically, MEK4 is overexpressed in advanced prostate cancer (PCa) lesions where it also displays pro-invasion/pro-metastatic activity promoting the cancer spread to distant locations.
Preventing metastasis is one of the key therapeutic goals of cancer treatment, hence inhibiting MEK4 pathway is a desirable strategy to add to the ongoing efforts. However, no selective MEK4 inhibitors have been developed to date.
The Scheidt’s group at NU has come up with a novel computational method to solve the problem. The results are described in a recent issue of the Journal of Chemical Information and Modeling. The authors used a diverse set of 84 putative MEK4 inhibitors to perform inhibitor docking simulations using the structure-based quantitative structure-activity relationship (QSAR) modeling. By coupling the QSAR with the perturbations of electrostatic potential (ESP) charge densities of donor and acceptor atoms in the ligands and MEK4 binding site, they developed a novel and highly predictive alignment-independent 3D-QSAR model. The authors hope the described methodology will help guide and optimize any future high-throughput screens for compounds with MEK4 inhibitory activity.
Four NU scientists who are also past CBC Awardees (see below for their specific CBC affiliations) contributed to this study: Raymond Bergan (now at Oregon Health & Science University), Matt Clutter, Karl Scheidt and Gary Schiltz. Karl Scheidt, PhD, Professor of Chemistry at NU Weinberg College of Arts and Sciences and Pharmacology is senior author on the current study.
The publication acknowledges CBC support as part of the work carried out at the NU Center for Molecular Innovation and Drug Discovery (CMIDD) — a facility funded by the *CBC Lever Award (2009). This Award was made to Sergey Kozmin (UChicago), Karl Scheidt (NU) and Jie Liang (UIC) to support the establishment of the Chicago Tri-Institutional Center of Excellence in Chemical Methodologies & Library Development.
Congratulations to all authors involved in the published research!
Publication attributed to *CBC funding:
Mishra RK, Deibler KK, Clutter MR, Vagadia PP, O’Connor M, Schiltz GE, Bergan R, Scheidt KA. Modeling MEK4 Kinase Inhibitors Through Perturbed Electrostatic Potential (ESP) Charges. J Chem Inf Model. 2019 Oct 28;59(10):4460-4466. (PubMed)
ABSTRACT:
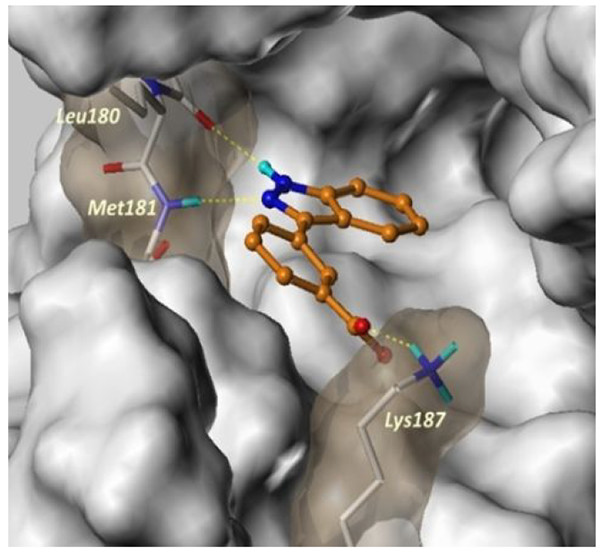
MEK4 ATP binding site.
(Source: J. Chem. Inf. Model. 2019, 59, 10, 4460-4466)
MEK4, mitogen-activated protein kinase kinase 4, is overexpressed and induces metastasis in advanced prostate cancer lesions. However, the value of MEK4 as an oncology target has not been pharmacologically validated because selective chemical probes targeting MEK4 have not been developed. With advances in both computer and biological high-throughput screening, selective chemical entities can be discovered. Structure-based quantitative structure-activity relationship (QSAR) modeling often fails to generate accurate models due to poor alignment of training sets containing highly diverse compounds. Here we describe a highly predictive, nonalignment based robust QSAR model based on a data set of strikingly diverse MEK4 inhibitors. We computed the electrostatic potential (ESP) charges using a density functional theory (DFT) formalism of the donor and acceptor atoms of the ligands and hinge residues. Novel descriptors were then generated from the perturbation of the charge densities of the donor and acceptor atoms and were used to model a diverse set of 84 compounds, from which we built a robust predictive model.
ACKNOWLEDGMENTS:
Financial support for this work was provided by the National Cancer Institute (NCI; R01 CA188015). K.K.D. thanks the US National Science Foundation (NSF) for a graduate research fellowship (DGE-1324585). A part of this work was performed by the Northwestern University Medicinal and Synthetic Chemistry Core (ChemCore) at the Center for Molecular Innovation and Drug Discovery (CMIDD), which is funded by the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust and Cancer Center Support Grant P30 CA060553 from the NCI awarded to the Robert H. Lurie Comprehensive Cancer Center.
Featured CBC Community member(s):
Raymond Bergan, **Oregon Health & Science University (OHSU)
**At NU at the time of the award
- CBC HTS Award (2013):
▸ A HTS Strategy to Identify and Validate Inhibitors of Mitogen-activated Protein Kinase Kinase 4
PIs: Raymond Bergan (OHSU; at NU at the time of the award), Karl Scheidt (NU) and Sankar Krishna (NU)
Matt Clutter, NU
- CBC HTS Award (2013):
▸ Identification of Selective mTORC2 Inhibitors for the Treatment of Cancer
PIs: Elspeth Beauchamp, Matt Clutter and Leonidas Platanias (NU)
Karl Scheidt, NU
- CBC Postdoctoral Research Award Reviewer (2014-2016):
Karl Scheidt (NU) — Reviewer - 11th Annual CBC Symposium (2013):
▸ Exploring Human Biology with Small Molecules
Karl Scheidt (NU) — Symposium Organizer - CBC HTS Award (2013):
▸ A HTS Strategy to Identify and Validate Inhibitors of Mitogen-activated Protein Kinase Kinase 4
PIs: Raymond Bergan (OHSU; at NU at the time of the award), Karl Scheidt (NU) and Sankar Krishna (NU) - CBC Tech Day (2012):
▸ Small Molecule Discovery in Academia
Karl Scheidt (NU) — Panelist - *CBC Lever Award (2009):
▸ Chicago Tri-Institutional Center of Excellence in Chemical Methodologies and Library Development
PIs: Sergey Kozmin (UChicago), Karl Scheidt (NU) and Jie Liang (UIC)
Gary Schiltz, NU
- CBC Accelerator Review Board (ARB) (2018-present):
▸ CBC Accelerator Review Board Current Membership
Gary Schiltz (NU) — Board Member - CBC HTS Award (2015):
▸ Identification of Protein Sequestration Inhibitors for Treating ALS
PIs: Gary Schiltz and Richard Silverman (NU)
ARTICLES PUBLISHED IN THE PAST ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
July 12, 2019
▸ Simulation-based small molecule screen
Four NU researchers and past CBC Awardees, Leonidas Platanias, Elspeth Beauchamp, Matt Clutter and Gary Schiltz, publish research funded in part by a CBC Lever Award
May 14, 2019
▸ Developing sigma-2 specific probes and therapeutics
CBC Awardee Gary Schiltz, NU, contributes; CBC acknowledged for partial funding of the work recently published in ChemMedChem
May 11, 2019
▸ Will mTORC + cTORC be “it”?
Four CBC affiliates contribute to a new discovery of another TORC protein complex which promises to become a therapeutic target for some of the treatment resistant leukemias
September 28, 2018
▸ Halting cancer metastasis
CBC affiliate Karl Scheidt, NU, and senior co-author on the publication in Nature Communications interviewed for Chicago Crain’s Business
June 27, 2018
▸ KBU2046—new compound that inhibits cancer cell motility
January 4, 2018
▸ New molecule with promise to combat multiple myeloma discovered in NU center established with CBC support
November 23, 2017
▸ CBC’s mission of collaboration embraced through inter-institutional teaching and mentoring
October 29, 2017
▸ Embracing collaboration to combat glioblastoma
Spotlight on the Northwestern University researchers and nanotechnology