April 2, 2019 | Jola Glotzer
Divertin may stop the…unstoppable
Two CBC Catalyst Awardees, Jerrold Turner and Lawrence Miller, describe Divertin — a novel molecule that effectively stops diarrhea and other symptoms of inflammatory bowel disease (IBD) in animal models
Congratulations to a CBC Catalyst Team, Jerrold Turner and Lawrence Miller, for their recent publication in Nature Medicine, “Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis.” The authors acknowledge partial funding via the CBC Catalyst Award (2008). At the time of the award, Turner was Professor of Pathology at UChicago; currently he is Professor of Pathology and Medicine, Harvard Medical School and Brigham and Women’s Hospital. Miller is Associate Professor at the Department of Chemistry, UIC. The Nature Medicine paper describes identification of a small molecule which they call Divertin, that specifically targets MLCK1 – an enzyme critical in regulation of the gut epithelial barrier. In animal models of IBD and diarrhea, Divertin restored the dysfunctional barrier, significantly reducing otherwise crippling symptoms. More studies are needed but as is, Divertin appears to have promise as a therapeutic agent to be tested in several types of human disease that are triggered by malfunctioning epithelial barrier. As a side-note — it is remarkable to see the CBC’s impact on biomedical discovery 11 years past the time of the award. Congratulations to all authors involved in this important and clinically promising study.
New therapeutic strategy to prevent gastrointestinal disease
New approach restores epithelial function in diarrhea and inflammatory bowel disease models
EurekAlert! | BRIGHAM AND WOMEN’S HOSPITAL Press release by Mark Murphy | April 1, 2019
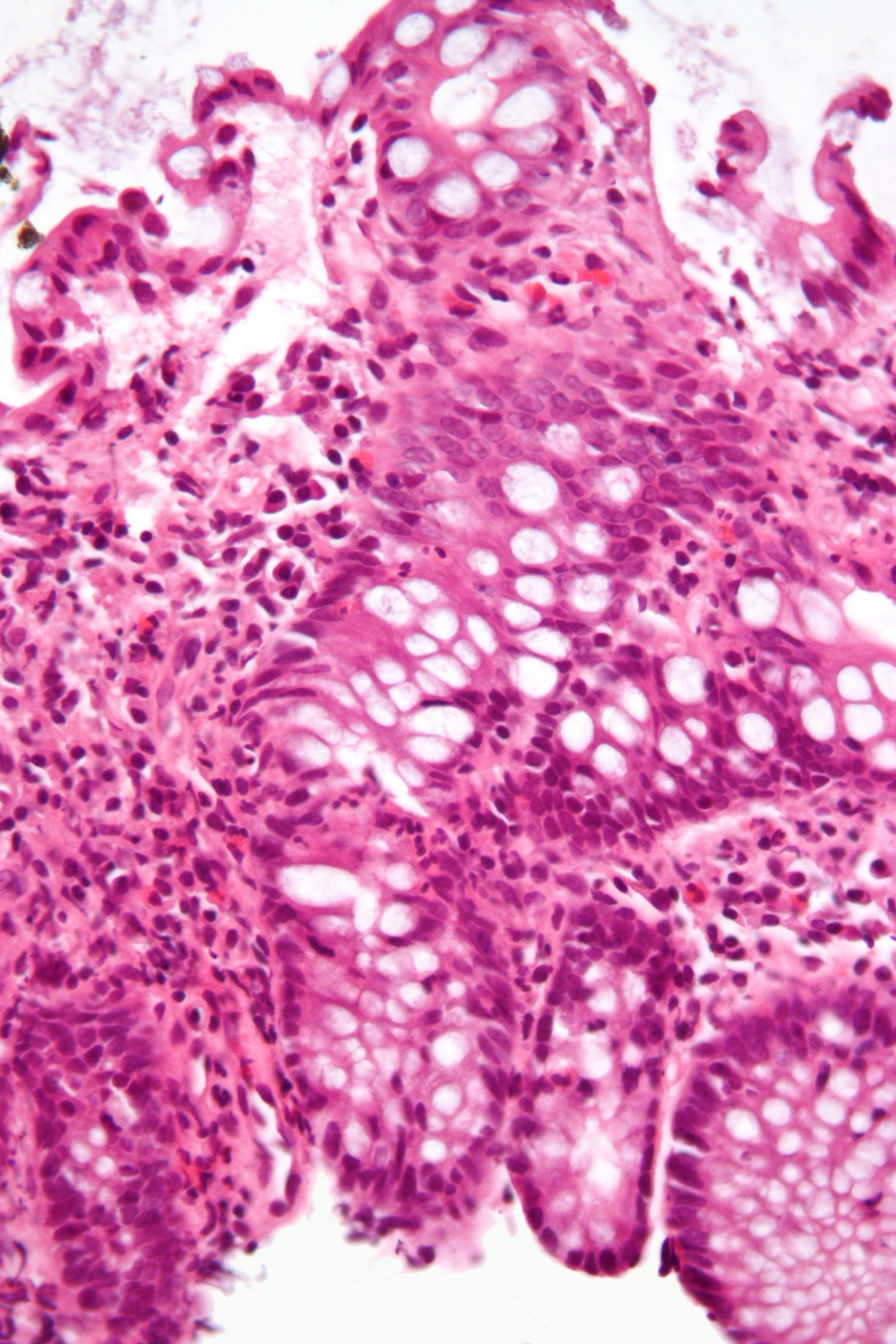
Micrograph showing inflammation of the large bowel in a case of inflammatory bowel disease. Colonic biopsy. (Credit: Wikipedia/CC BY-SA 3.0.)
Food allergies, celiac disease, inflammatory bowel disease (IBD), diarrhea and other gastrointestinal diseases have something in common: all have been linked to epithelial barrier loss. The gut epithelial barrier–that critical lining of cells in the gut that must allow nutrients into the body while keeping food-borne microbes out–can be compromised during intestinal inflammation and cause disease. While many of the molecular mechanisms that trigger gastrointestinal diseases remain a mystery, previous research has found that one enzyme, known as myosin light chain kinase (MLCK), plays a critical role. However, MLCK is also essential for critical functions in gut epithelia and other cell types. This makes direct inhibition of MLCK impossible, as it would result in many toxic and systemic side effects. A team led by investigators from Brigham and Women’s Hospital has now developed an alternative approach. In a study published in Nature Medicine, the researchers report new evidence suggesting that specifically targeting one version of the enzyme–MLCK1–may be effective in both preventing and treating gastrointestinal disease by preserving and restoring barrier function, respectively.
“This represents a completely novel, non-toxic approach to intestinal barrier restoration and treatment of inflammatory bowel disease,” said corresponding author Jerrold Turner, MD, PhD, of the Department of Pathology at the Brigham.

Jerrold Turner, MD, PhD, of the Department of Pathology at the Brigham. (Source: Turner Lab Homepage)
Other forms of MLCK can be found throughout the gut lining, in various epithelia and in smooth muscle. But the MLCK1 isoform is particularly expressed in the villous enterocytes–intestinal cells that sit in the finger-like projections that extend into the lumens of the small intestine–and corresponding surface cells of the colon. To lay the groundwork for targeting only MLCK1, Turner and his team solved the crystal structure of the region that distinguishes MLCK1 from other variants. They then used computer modeling to screen approximately 140,000 molecules, looking for one that could dock into this region like a key in a lock.
The team found a molecule, which they named Divertin, that fit neatly into this pocket. Divertin (so-named because it diverts MLCK1 away from the intracellular sites at which it regulates the barrier) prevented inflammation-induced barrier loss without compromising key MLCK enzymatic functions in epithelia and smooth muscle. In mouse models of inflammatory bowel disease and diarrhea, Divertin corrected barrier dysfunction and reduced disease severity. When given prophylactically, Divertin prevented disease development and progression. This suggests that Divertin might be a new, non-immunosuppressive means to maintain remission, and prevent disease flares, in patients with IBD.
Turner and colleagues note that targeting MLCK1 to prevent barrier loss and restore function could also be useful in other diseases where the epithelial barrier is compromised, including celiac disease, atopic dermatitis, pulmonary infection and acute respiratory distress syndrome, multiple sclerosis, and graft versus host disease (GVHD). In a study published recently in The Journal of Clinical Investigation, the same research team presented evidence that MLCK drives the continuation of GVHD, a complication that can arise after a transplant when donor immune cells begin attacking the organs of a transplant recipient. While Divertin was not tested in GVHD, the data suggest that it may be an effective therapy.

Lawrence Miller, PhD, Associate Professor of Chemistry, UIC (Source: Miller Lab Homepage)
“Our study indicates that MLCK1 is a viable target for preserving epithelial barrier function in intestinal diseases and beyond,” said Turner. “This therapeutic approach may help break the cycle of inflammation that drives so many chronic diseases.”
Turner and Lawrence Miller, PhD, Associate Professor of Chemistry, UIC, are co-senior authors on the Nature Medicine publication that was partially supported by a CBC Catalyst Award, won by the team in 2008 while Turner was a Professor at UChicago.
Funding for this work was provided by the National Institutes of Health (R01DK61931, R01DK68271, P30CA14599, P30DK034854, and T32HL007237), the Broad Medical Research Foundation (IBD-022), the Department of Defense (W81XWH-09-1-0341), a Catalyst Award from the Chicago Biomedical Consortium, and the National Natural Science Foundation of China (81470804 and 31401229). The Berkeley Center for Structural Biology is supported in part by the National Institutes of Health, National Institute of General Medical Sciences, and the Howard Hughes Medical Institute. The Advanced Light Source is a Department of Energy Office of Science User Facility under Contract No. DE-AC02-05CH11231.
Publication attributed to the CBC funding*:
Graham WV, He W, Marchiando AM, Zha J, Singh G, Li HS, Biswas A, Ong MLDM, Jiang ZH, Choi W, Zuccola H, Wang Y, Griffith G, Wu J, Rosenberg HJ, Wang Y, Snapper SB, Ostrov D, Meredith SC, Miller LW, Turner JR. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nature Medicine. Published: 01 April 2019. (nature)
Source:
Adapted (with modifications) from the EurekAlert!, by Mark Murphy, published on April 1, 2019.
Featured CBC Community member(s):
Jerrold Turner, Harvard & Brigham and Women’s Hospital; at UChicago at the time of the award
- CBC Workshop (2011):
▸ CBC Catalyst Award program
Jerrold Turner (UChicago) — Speaker - CBC Scholar Award (2010-2011):
▸ Meet the Scholar: Sam Nalle
Jerrold Turner (UChicago) — Scholar’s Mentor - CBC Forum (2009):
▸ Microscopy Forum: Seeing Small is Believing Big
Jerrold Turner (UChicago) — Forum Organizer and Speaker - *CBC Catalyst Award (2008):
▸ Multiplexed Imaging of Transient Molecular Complex Dynamics in vivo
PI(s): Jerrold Turner (UChicago) and Lawrence Miller (UIC) - CBC Spark Review Panel:
Jerrold Turner (UChicago) — Reviewer
Lawrence Miller, UIC
- CBC Catalyst Review Board (2017—present):
▸ Current Membership
Lawrence Miller (UIC) — Member - CBC Workshop (2013):
▸ CBC Catalyst Award program
Lawrence Miller (UIC) — Speaker - CBC Science Day (2011):
▸ CBC Science Day program
Lawrence Miller (UIC) — Speaker - CBC Forum (2009):
▸ Microscopy Forum: Seeing Small is Believing Big
Lawrence Miller (UIC) — Forum Organizer and Speaker - *CBC Catalyst Award (2008):
▸ Multiplexed Imaging of Transient Molecular Complex Dynamics in vivo
PI(s): Jerrold Turner (UChicago) and Lawrence Miller (UIC)
Related:
September 28, 2010
▸ CBC Catalyst Team Develops a Novel Technique to Visualize Protein Interactions in Living Cells
