August 29, 2019 | Jola Glotzer
Supplementing autologous bone grafts
A CBC Catalyst Team of Guillermo Ameer, NU, Tong-Chuan He and Russell Reid, UChicago, tests Imiquimod’s potential to induce differentiation of mesenchymal stem cells toward an osteogenic phenotype, with implications for bone reconstruction.
A CBC Catalyst Team (from the left): Guillermo Ameer (NU), Tong-Chuan He (UChicago), and Russell Reid (UChicago)
Congratulations to a CBC Catalyst team of Guillermo Ameer, NU, Tong-Chuan He and Russell Reid, UChicago, on their recent publication, “Imiquimod acts synergistically with BMP9 via the Notch pathway as an osteoinductive agent in vitro.” In the paper, a trio of the scientists acknowledges CBC support through a CBC Catalyst Award they received in 2013, for the project titled: “Craniofacial Tissue Engineering with Citric-Acid Based Nanocomposite Scaffolds.” The team has pursued a long-standing interest in reconstructive surgery that involves bones, however, such surgeries, which typically require autologous bone grafts, are frequently complicated by an insufficiency of bone material, especially in reconstructive surgeries requiring multiple grafts.
Artificial systems are being evaluated to address this problem, currently being tested to either support the autologous bone grafts with synthetic matrices, and/or by supplementing the grafts with mesenchymal stem cells (MSC), induced to differentiate towards the bone cell lineage.
In the current study, the Catalyst team and their collaborators tested a synthetic immune response modifier, Imiquimod, in stimulating the osteogenic differentiation of MSC. They find that Imiquimod appears to activate Notch signaling and synergizes with BMP9 in stimulating osteogenesis. These findings may have a therapeutic potential to be explored to help with bone reconstructive surgeries.
Guillermo Ameer, DSc, is Daniel Hale Williams Professor of Biomedical Engineering, Professor of Surgery, and Director of the Center for Advanced Regenerative Engineering, NU. Tong-Chuan He, MD, PhD, and Russell R. Reid, MD, PhD, work at the Department of Surgery, UChicago. Dr. He is Associate Professor of Surgery, and Associate Professor of Orthopedic Surgery and Rehabilitation Medicine, whereas Dr. Reid is Professor of Surgery, specializing in Pediatric Plastic & Reconstructive Surgery, and Bernard Sarnat Scholar of Craniofacial Research.
Other ties to the CBC of the three CBC Catalyst team members are listed below.
Publication attributed to the CBC funding*:
Alverdy AK, Pakvasa M, Zhao C, Mostafa S, Liu W, Luo W, Wolf JM, Ameer GA, He TC, Reid RR. Imiquimod acts synergistically with BMP9 via the Notch pathway as an osteoinductive agent in vitro. Plast Reconstr Surg. 2019 Aug 1. [Epub ahead of print] (PubMed)
ABSTRACT:
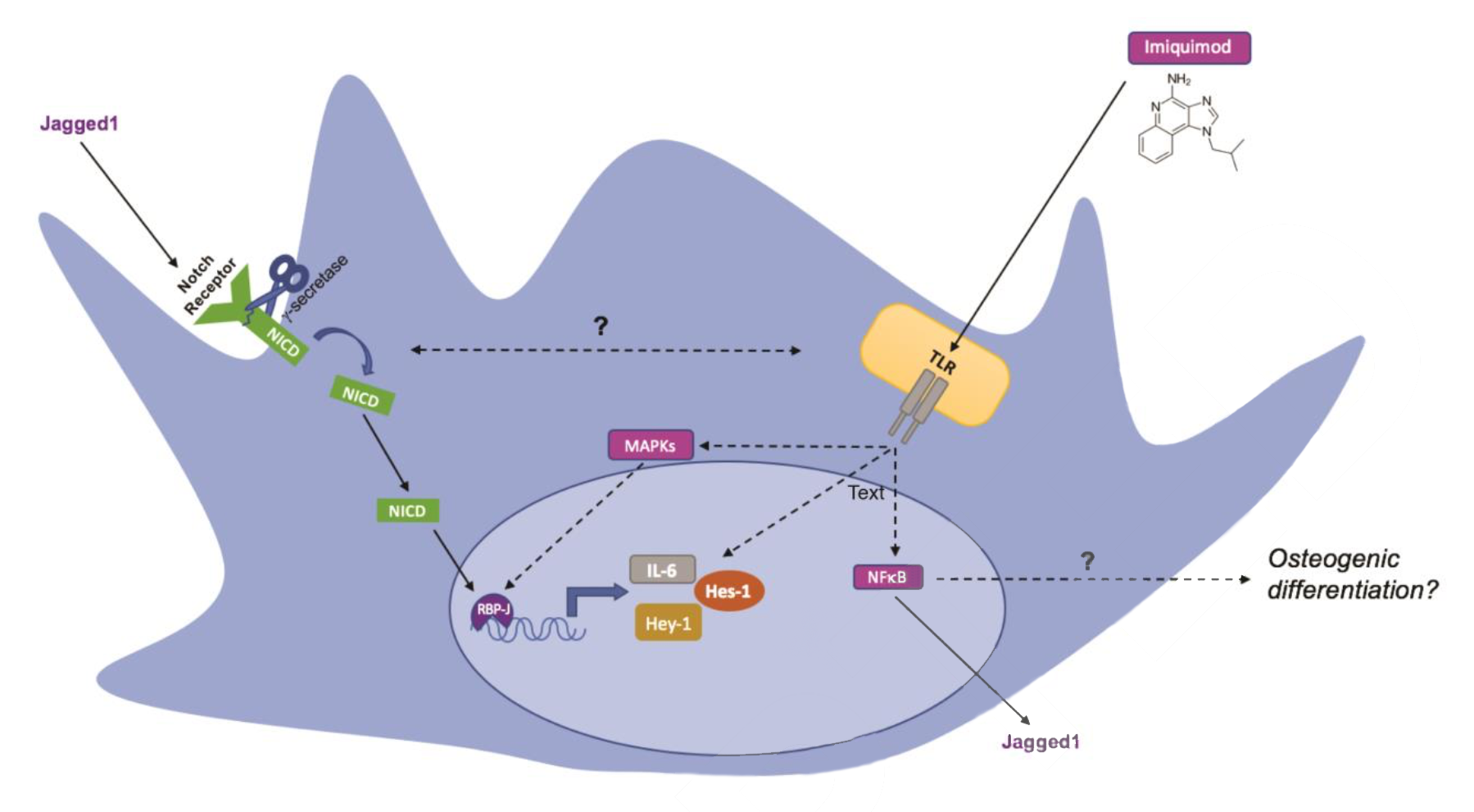
Schematic presentation of potential pathways in Imiquimod-induced osteogenic differentiation. (Source: insights.ovid.com)
BACKGROUND:
Autologous bone grafts used for surgical reconstruction are limited by infection or insufficient supply of host material. Experimental agents that promote differentiation of stem cells into mature bone are currently being studied for future use in the repair of bone defects. We hypothesized that Imiquimod, a synthetic immune response modifier, increases Notch pathway gene expression and acts synergistically with BMP9 to induce differentiation of mesenchymal stem cells (MSCs) toward an osteogenic phenotype.
METHODS:
Alkaline phosphatase (ALP) activity was used to assess osteogenic potential of cultured mouse multipotent adipose-derived cells (iMADs) treated with 0, 4, 6, and 8 ∞g/mL of Imiquimod with and without BMP9. Adenoviral vectors expressing human-BMP9 and a dominant-negative mutant of mouse Notch1 were used to assess BMP9 and Notch blockade, on osteogenic activity, respectively. Expression of Notch signaling mediators and osteogenic markers were assayed by qPCR. Alizarin Red staining was used to assess the synergism between BMP9 and Imiquimod.
RESULTS:
Imiquimod exposure enhanced osteogenic differentiation of iMADs by 2.8 fold (p<.001) and potentiated BMP-9 induced osteogenic differentiation of iMADs by 1.6 fold (p<.001), shown by increased ALP activity and augmented matrix mineralization. Quantitative-real time PCR analysis demonstrated that Imiquimod induced the expression of downstream genes (p<.01) of the Notch signaling pathway Hey1, Hey2, and Hes1, by a fold increase of 9.7, 22, and 2.7, respectively.
CONCLUSIONS:
These findings identify a novel role for Imiquimod to shift MSCs toward an osteogenic phenotype. Imiquimod may be useful clinically when scaffolds are applied to treat bone defects.
ACKNOWLEDGMENTS:
The reported work was supported in part by research grants from the National Institutes of Health (CA226303, DE020140 to TCH and RRR), the U.S. Department of Defense (OR130096 to JMW), the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (RRR, TCH and GAA), the Scoliosis Research Society (TCH and MJL), and the National Key Research and Development Program of China (2016YFC1000803 and 2011CB707906 to TCH). Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Featured CBC Community member(s):
Tong-Chuan He and Russell Reid, UChicago
- *CBC Catalyst Award (2013):
▸ Craniofacial Tissue Engineering with Citric-Acid Based Nanocomposite Scaffolds
PIs: Tong-Chuan He (UChicago), Russell Reid (UChicago) and Guillermo Ameer (NU)
Guillermo Ameer, NU
- ▸ Inaugural CBCAN meeting (March 30, 2017):
Guillermo Ameer – Invited speaker - CBC Postdoctoral Research Award (2015):
▸ Synergistic Copper Metal-Organic Framework-Hydrogel Composite Improves Wound Healing
PIs: Josheng Xiaoand (postdoc) and Guillermo Ameer (NU) - CBC Postdoctoral Research Award (2014):
▸ Induced Pluripotent Stem Cells Derived Endothelial Cells in Vascular Tissue Engineering
PIs: Bin Jiang (postdoc), Guillermo Ameer and Jason Wertheim (NU) - CBC Postdoctoral Research Award (2014):
▸ Periadventitial delivery of retinoic acid via thermoresponsive hydrogel
PIs: Jian Yang (postdoc) and Guillermo Ameer (NU) - *CBC Catalyst Award (2013):
▸ Craniofacial Tissue Engineering with Citric-Acid Based Nanocomposite Scaffolds
PIs: Russell Reid and Tong-Chuan He (UChicago), and Guillermo Ameer (NU) - CBC Business Plan Competition (2009):
Winner: VesselTek Biomedical, an innovative vascular products company started by Guillermo Ameer
▸ An NU Start-Up Wins 2009 CBC Business Plan Competition
Articles published in the past about the featured CBC community members:
He & Reid
May 30, 2019
▸ Dissecting the roles of 14(!) BMPs
A CBC Catalyst Award to Tong-Chuan He and Russell Reid, UChicago, contributes to understanding the mechanisms behind bone and other mesenchyme-derived tissues differentiation
Reid
February 6, 2019
▸ UChicago named professorships
Three UChicago faculty with links to CBC receive named, distinguished service professorships: Robert Grossman, Russell Reid and Margaret Gardel
Ameer
April 14, 2019
▸ If you ‘jerk,’ you’ll die…
Four past CBC Awardees from Northwestern contribute to this fascinating Nature Communications publication: Andrew Stephens, John Marko, Guillermo Ameer and Vadim Backman
September 17, 2018
▸ A liquid bandage?
Developing new bandage with healing properties — Fox 32 Chicago News interview with CBC awardee, Guillermo Ameer, NU
June 18, 2018
▸ A wound-healing bandage
December 1, 2017
▸ Guillermo Ameer, NU bioengineer and CBC awardee, discusses the future of regenerative engineering
November 6, 2017
▸ Guillermo Ameer, NU bioengineer and CBC awardee, elected fellow of AIChE
April 7, 2017
▸ The CBC Accelerator Network (CBCAN) off to a great start!
