March 26, 2018
New, cell-free protein manufacturing methodology, developed by Michael Jewett’s NU team and supported by CBC funding, could have important implications in biomedicine and beyond
Congratulations to Michael Jewett, NU, who is senior author on a recent publication in Nature Communications! For a long time, Jewett and his team have been interested in scaling up and optimizing protein production – a process essential to generating large quantities of biomaterials including vaccines and biotherapeutics. Traditional methods of mass protein production process have relied upon living cells. Now, the Jewett lab has devised an artificial “protein factory” or a cell-free protein synthesis (CFPS) platform, that can synthesize vast amounts of protein of choice outside of a living organism. The CFPS platform opens doors to a new era of chemistry and protein manufacturing, with many implications including medicine. The publication acknowledges CBC funding to Jewett, via a CBC Catalyst Award he received in 2013. Other co-authors on the paper with links to CBC are: Neil Kelleher, Roderick Davis and Paul Thomas, NU. Congratulations to all researchers involved in the study!
New Innovations in Cell-free Biotechnology
New platform could lead to improved production of protein therapeutics and biomaterials
Northwestern University Engineering News | by STEPHAN BENZKOFER | March 23, 2018
A Northwestern University-led team has developed a new way to manufacture proteins outside of a cell that could have important implications in therapeutics and biomaterials.
The advance could make possible decentralized manufacturing and distribution processes for protein therapeutics that might, in the future, promote better access to costly drugs all over the world.
The team set out to improve the quality of manufactured proteins in vitro, or outside a cell, and found success across a number of fronts.
“We developed a bacterial cell-free protein synthesis system that is capable of high level expression of pure proteins containing multiple non-canonical amino acids,” said Michael Jewett, associate professor of chemical and biological engineering at Northwestern’s McCormick School of Engineering. “This is important because it allows us to expand the range of genetically encoded chemistry incorporated into proteins in previously unattainable ways.”
The team, which brought together researchers from Northwestern, Yale University, and the Illinois Institute of Technology, reported its work in the journal Nature Communications on March 23.
Protein production plays a critical role in medicine, biotechnologies, and life sciences. Recombinant protein production, for example, has transformed the lives of millions of people through the synthesis of biopharmaceuticals, like insulin, and industrial enzymes, like those used in laundry detergents. Conventionally, protein production has been accomplished in living cells in large centralized manufacturing facilities.
“Alternatively, what cell-free protein synthesis does is take the cell, rip off the cell wall, and collect the guts of the cell. We then use this to make a protein without a living, intact organism,” said Jewett, who is also co-director of Northwestern’s Center of Synthetic Biology. “You can imagine I’ve taken a car and opened the hood and taken out the engine and put it over here in my driveway. Now I can use it to do something else.”
Without the worry of trying to keep a cell alive, this process opens up many possibilities, including the synthesis of new classes of enzymes, therapeutics, materials, and chemicals with diverse chemistry. A living cell may balk when asked to do something it hasn’t seen in its evolutionary biology, not so for a cell-free protein synthesis (CFPS) platform.
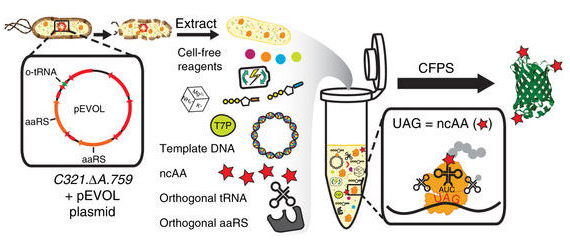
Cell-free protein synthesis (CFPS) from extracts of a genomically recoded organism. Schematic of the production and utilization of crude extract from genomically recoded organisms with plasmid overexpression of orthogonal translation components for CFPS. CFPS reactions are supplemented with the necessary substrates (e.g., amino acids, NTPs, etc.) required for in vitro transcription and translation as well as purified orthogonal translation system (OTS) components to help increase the ncAA incorporation efficiency. aaRS, aminoacyl tRNA synthetase; ncAA, non-canonical amino acid; T7P, T7 RNA polymerase; UAG, amber codon. Source: Nat Commun. 2018 Mar 23;9(1):1203.
The problem up to this point, though, has been that efforts to use CFPS systems for expanding multiple non-canonical amino acids have been limited by competition with the natural machinery that terminates protein synthesis. As a result, manufacturing proteins harboring diverse chemistries with high purity and yields has presented a formidable challenge.
But Jewett and the team produced the highest yields of proteins with non-canonical amino acids ever reported for in vitro systems, suggesting that long-term commercial applications for CFPS might be realistic.
Jewett credited the breakthrough to two elements. The first was the idea of using a genomically recoded organism of Escherichia coli bacteria that lacked release factor 1.
“This is important because it provided us an open coding channel to incorporate new chemistry,” he said. “Trying to develop a cell-free protein synthesis from that strain had never been done before.”
The second element came courtesy of Jewett’s student, Rey Martin, the lead author of the paper. Jewett said when they first tried to use the strain, they failed to make enough protein.
“What my student Rey did in a very innovative strategy was find genes in the chromosome of that organism that we thought were negatively affecting our ability to produce protein,” Jewett said. “He functionally inactivated them to enable higher cell-free protein synthesis yields.”
Jewett said that Martin didn’t stop there.
“Rey optimized the cell-free environment to enable multiple identical non-canonical amino acids to be incorporated,” Jewett said. Where before researchers have used just one or a few instances, he said his team was able to incorporate up to 40, site specifically with no observable truncation products.
“What that means is we can really change fundamentally the properties of protein polymers in unique and new ways,” Jewett said. “And you may ask, ‘well, what are you going do with that?’ From a research perspective, we haven’t had this exact capability before so we haven’t been able to even ask the question.”
Jewett imagines applications not just with medicines but also biomaterials for drug delivery systems, medical materials such as surgical sutures, and functionalized biopolymers.
“What’s special about this platform is that it’s already being used in so many other project areas in the lab,” Jewett said. “It has the potential to open up an entirely new area of materials chemistry research for biotechnology and, more broadly, could enable new paradigms of on-demand biomanufacturing of vaccines and therapeutics.”
Source:
Adapted (with modifications) from Northwestern University Engineering News, by Stephan Benzkofer, posted on March 23, 2018.
*Publication attributed to CBC funding:
Martin RW, Des Soye BJ, Kwon YC, Kay J, Davis RG, Thomas PM, Majewska NI, Chen CX, Marcum RD, Weiss MG, Stoddart AE, Amiram M, Ranji Charna AK, Patel JR, Isaacs FJ, Kelleher NL, Hong SH, Jewett MC. Cell-free protein synthesis from genomically recoded bacteria enables multisite incorporation of noncanonical amino acids. Nat Commun. 2018 Mar 23;9(1):1203. (PubMed)
Jewett, Kelleher, Thomas and Davis have the following ties to CBC:
- *Catalyst Review Board (CRB) (2016 – present):
Michael Jewett (NU) – Board Member - 12th Annual CBC Symposium (2014):
▸ Protein Engineering: From Computers to Cells to Clinic
Michael Jewett (NU) – Symposium Speaker - *CBC Catalyst Award (2013):
▸ Engineering Prokaryotic Translation for Artificial Metalloenzyme Production
PIs: Michael Jewett (NU) and Jared Lewis (UChicago) - CBC Scholars “Loop Connections” Seminar (2013):
▸ Establishing Cell-Free Biology for the Production of Therapeutics, Materials, and Chemicals
Michael Jewett (NU) – Seminar Speaker - CBC Exploratory Workshop (2013):
▸ The CBC Exploratory Workshop on Cellular Heterogeneity
Neil Kelleher (NU) – Workshop Organizer and Speaker - CBC Science Day (2011):
Neil Kelleher (NU) – Science Day Speaker - CBC Catalyst Award (2010):
▸ Phosphoproteomic Analysis of NADPH Oxidase Activation
PIs: Neil Kelleher (NU) and Richard Ye (UIC) - CBC Recruitment Resources Award (2010):
▸ CBC Senior Investigator
Neil Kelleher (NU) - CBC Seminar (2010):
▸ Top Down Mass Spectrometry: Has Its Decade Now Come?
Neil Kelleher (NU, UIUC then) – Seminar Speaker - CBC Summer Workshops in Proteomics and Informatics (2010, 2011, 2012, 2013, 2014, 2015)
Paul Thomas (NU) – Workshop Lecturer - CBC Summer Workshops in Proteomics and Informatics (2010, 2011, 2012, 2013, 2014)
Roderick Davis (NU, at UIC then) – Workshop Lecturer

