July 26, 2019 | Jola Glotzer
Embryonic stem cell defect in autism
A CBC Catalyst Award team of Yongchao Ma, NU and Chuan He, UChicago, discover how the embryonically-expressed protein FMRP, when mutated, inhibits stem cell differentiation into embryonic brain neurons thus causing the development of fragile X syndrome, a form of autism
Fragile X syndrome — a type of autism — is the most common inherited intellectual disability in children. Previous studies have pinpointed a culprit gene, called FMR1, which plays a critical role in causing slower brain development in children carrying the FMR1 mutations as compared to the healthy population. In 2016, Yongchao Ma, NU, who’s lab investigates the regulation of neuron function in development and disease, joined forces with Chuan He, UChicago, a world expert in uncovering the role that dynamic and reversible RNA and DNA methylation play in gene expression regulation. Ma and He won a CBC Catalyst Award — given for novel, high-risk/high-reward projects with transformative potential — and the results of this collaborative work have recently been published in Cell Reports.
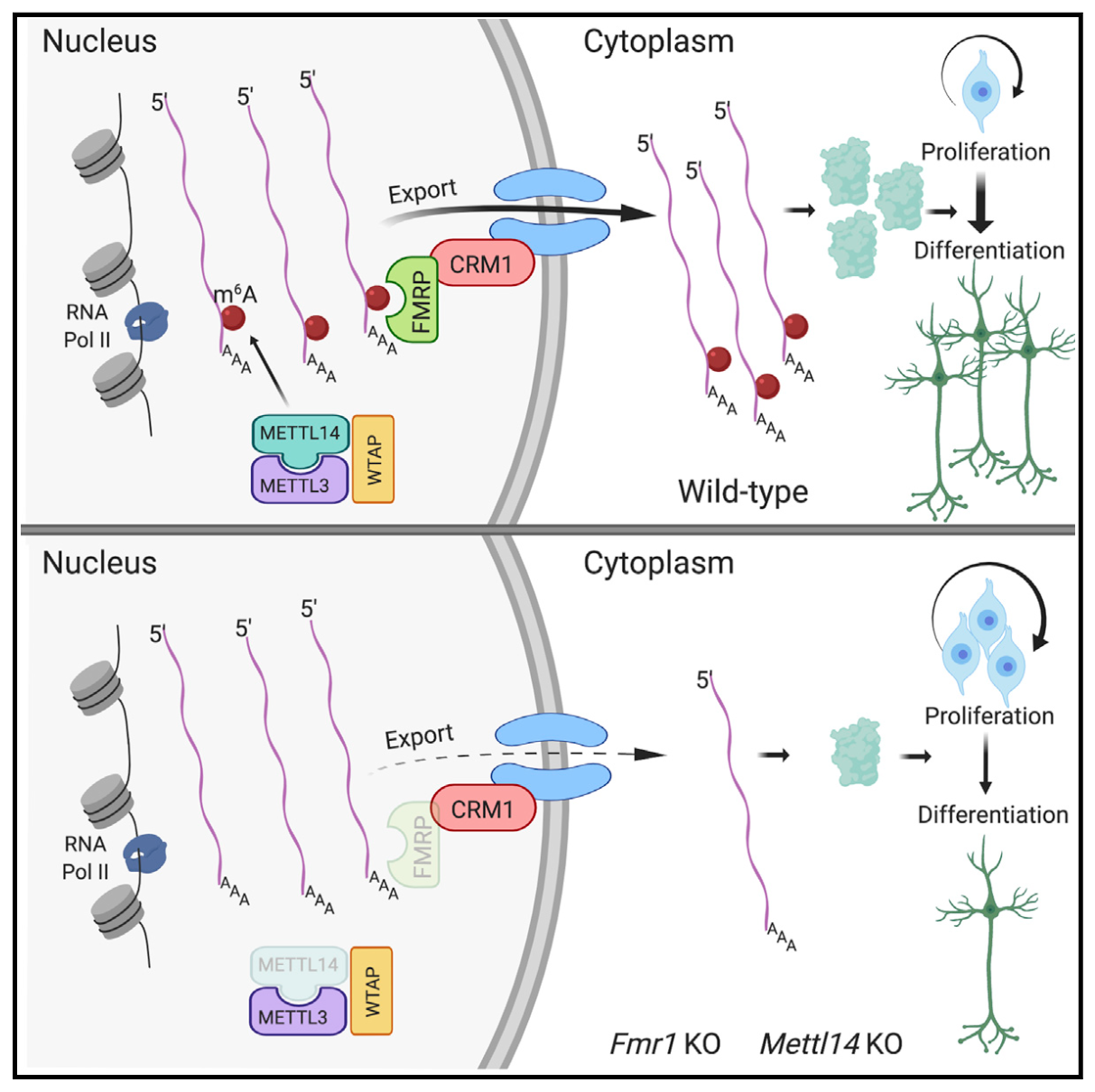
The Catalyst team discovered that the FMR1-encoded protein FMRP acts as a “reader” of the methylation of RNA on N6-adenosine, called m6A. FMRP facilitates transport of m6A-modified RNA form the nucleus to the cytoplasm where the RNA is subsequently translated into a protein. However, if FMRP is mutated or missing, as seen in fragile X syndrome, m6A-RNA cannot leave the nucleus. Such nuclear accumulation of many mRNAs encoding proteins that are critical for proper differentiation of stem cells into neurons in the developing embryonic brain results in developmental delays, as observed in a mouse model.
These important new findings shed new light on the mechanisms that hinder proper differentiation of neurons and growth of the embryonic brain. A natural question could be raised whether targeted gene therapy could one day help overcome the “molecular block” and nip in the bud the development of autism and other diseases that depend on FMRP interactions with m6A-RNAs.
The CBC congratulates the CBC Catalyst team and all other authors involved in the current study and appreciates being recognized for partially funding the published work.
Study Looks at Stem Cells for Answers to How a Type of Autism Develops
Investigating the earliest stages in development of fragile X syndrome offers new targets for potential treatments
Ann & Robert H. Lurie Children’s Hospital of Chicago News and Stories | News Release | by Vita Lerman, | July 23, 2019


A CBC Catalyst Awardee, Yongchao Ma, PhD, (left) associate professor of Pediatrics and a member of Stanley Manne Children’s Research Institute at Ann & Robert H. Lurie Children’s Hospital of Chicago was the principal investigator of the study published in Cell Reports. (Courtesy of NU Medicine News.) Ma’s CBC Catalyst Award team member, Chuan He, PhD, John T. Wilson Distinguished Service Professor in the Department of Chemistry, The University of Chicago, is co-author on the publication. (Courtesy of UChicago Department of Chemistry.)
The lab of Yongchao Ma, PhD, from Stanley Manne Children’s Research Institute at Ann & Robert H. Lurie Children’s Hospital of Chicago, discovered how the genetic defect in fragile X syndrome – a type of autism – delays production of neurons (nerve cells) at a critical time in the embryo’s brain development. In a study published in Cell Reports, Dr. Ma and colleagues describe a previously unknown regulatory mechanism controlling how stem cells differentiate into neurons. They identified early disruptions in this process in fragile X syndrome, the most common inherited intellectual disability in children.
“During embryonic brain development, the right neurons have to be produced at the right time and in the right numbers,” says Dr. Ma, senior author and researcher at Lurie Children’s, as well as Associate Professor of Pediatrics, Neurology and Physiology at Northwestern University Feinberg School of Medicine. “We focused on what happens in the stem cells that leads to slower production of neurons that are responsible for brain functions including learning and memory. Our discoveries shed light on the earliest stages of disease development and offer novel targets for potential treatments.”
Other studies in fragile X development have focused on the interactions between mature neurons. Dr. Ma’s study is the first to offer a new understanding of the disease at a stem cell level.
Fragile X syndrome occurs in approximately 1 in 4,000 males and 1 in 8,000 females. It is caused by mutation in the gene called FMR1 that encodes a protein called FMRP. The genetic defect leads to reduced FMRP protein. Previously the function of FMRP protein during early brain development was not known.
Graphical Abstract. (Source: Cell Reports.)
Dr. Ma and colleagues discovered that within a stem cell, the FMRP protein plays a key role as a “reader” of a chemical tag (called m6A) on the RNA. This tag carries instructions on how to process the RNA. By reading these instructions, FMRP protein exports the RNAs from the nucleus to the cytoplasm of cells where the m6A-tagged RNAs will become proteins that control stem cell differentiation into neurons.
“We show how the reduced amount of FMRP protein in neural stem cell results in decreased nuclear export of m6A-tagged RNAs and ultimately, slower production of the neurons that are essential for healthy brain development,” says first author Brittany Edens, graduate student in the Northwestern University Interdepartmental Neuroscience Program who works in Dr. Ma’s lab. “Our findings also expand understanding of how the flow of genetic information form DNA to RNA to protein is regulated, which is a central question in biology.”
“Currently we are exploring how to stimulate FMRP protein activity in the stem cell, in order to correct the timing of neuron production and ensure that the correct amount and types of neurons are available to the developing brain,” says Dr. Ma. “There may be potential for gene therapy for fragile X syndrome.”
Chuan He, PhD, John T. Wilson Distinguished Service Professor in the Department of Chemistry, The University of Chicago, is co-author on the publication.
ACKNOWLEDGMENTS:
This research was supported by grants from the NIH (R01NS094564 and R21NS106307 to Y.C.M.; R37NS047344, U19MH106434, and P01NS097206 to H. Song; RM1HG008935 to C.H.; and R01MH105128, R35NS097370, and U19AI131130 to G.-l.M.); the Simons Foundation Autism Research Initiative (SAFRI) to H. Song (575050); Cure SMA and The Hartwell Foundation (to Y.C.M.); and the Chicago Biomedical Consortium (to Y.C.M. and C.H.). C.V. was partially supported by an NSF predoctoral fellowship and NIH T32GM007445. We thank Dr. Stephanie Ceman for providing WT and ΔNES Fmr1 constructs, Dr. Anis Contractor for providing Fmr1 KO mice, and Dr. Xiaoxi Zhuang for sharing Mettl14f/f mice. We thank Dr. Tian Shao for technical assistance in validating anti-FMRP antibodies. The research reported in this manuscript was made possible in part by the services of the Keck Biophysics Facility and the NUSeq Core Facility, which is supported by the Northwestern University Center for Genetic Medicine, the Feinberg School of Medicine, and the Shared and Core Facilities of Northwestern University’s Office for Research. C.H. is a Howard Hughes Medical Institute Investigator. Y.C.M. is the Ann Marie and Francis Klocke M.D. Research Scholar supported by the Joseph and Bessie Feinberg Foundation.
About the Ann & Robert H. Lurie Children’s Hospital of Chicago
Research at Ann & Robert H. Lurie Children’s Hospital of Chicago is conducted through the Stanley Manne Children’s Research Institute. The Manne Research Institute is focused on improving child health, transforming pediatric medicine and ensuring healthier futures through the relentless pursuit of knowledge. Lurie Children’s is ranked as one of the nation’s top children’s hospitals by U.S. News & World Report. It is the pediatric training ground for Northwestern University Feinberg School of Medicine. Last year, the hospital served more than 212,000 children from 49 states and 51 countries.
Source:
Adopted (with modifications) from Ann & Robert H. Lurie Children’s Hospital of Chicago News and Stories, by Vita Lerman, July 23, 2019.
See also:
▸ New Insights Into Role of RNA Methylation in Fragile X Syndrome
by Anna Williams, Northwestern Medicine News, July 23, 2019
Publication attributed to CBC funding*:
Edens BM, Vissers C, Su J, Arumugam S, Xu Z, Shi H, Miller N, Rojas Ringeling F, Ming GL, He C, Song H, Ma YC. FMRP Modulates Neural Differentiation through m6A-Dependent mRNA Nuclear Export. Cell Rep. 2019 Jul 23;28(4):845-854.e5. (PubMed)
Featured CBC Community member(s):
Yongchao Ma, NU
- *CBC Catalyst Award (2016):
▸ Deciphering RNA Methylation in Regulating Neuronal Functions in Health and Disease
PIs: Yongchao Ma (NU) and Chuan He (UChicago)
Chuan He, UChicago
- CBC Accelerator Award (2018):
▸ A Highly Sensitive and Robust Test for Early Colorectal Cancer Diagnosis
PI: Chuan He (UChicago) - CBC Accelerator Network (CBCAN) (February 12, 2018):
▸ CBC Accelerator Network Forum
Chuan He (UChicago) — Accelerator Award program LOI presenter and award finalist - CBC Accelerator Network (CBCAN) (April 7, 2017):
▸ CBC Accelerator Network Forum
Chuan He (UChicago) — CBCAN Speaker - *CBC Catalyst Award (2016):
▸ Deciphering RNA Methylation in Regulating Neuronal Functions in Health and Disease
PIs: Yongchao Ma (NU) and Chuan He (UChicago) - CBC Postdoctoral Research Award (2016):
▸ Novel Sequencing Method to Profile 5mC and 5hmC in Single Cell at Base Resolution
PIs: Lulu Hu (postdoc) and Chuan He (UChicago) - CBC Postdoctoral Research Award (2015):
▸ Novel Sequencing Method to Profile N6-Methyldeoxyadenosine in Single-base resolution
PIs: Guan-Zheng Luo (postdoc) and Chuan He (UChicago) - CBC Postdoctoral Research Award (2014):
▸ Screening of Small Molecule Inhibitors for Nuclear RNA M6a Methyltransferase Complex
PIs: Jianzhao Liu (postdoc) and Chuan He (UChicago) - CBC Postdoctoral Research Award (2014):
▸ Pseudouridine Sequencing – A Roadmap to the New Biology of Mrna Pseudouridine Modification
PIs: Dan Dominissini (postdoc) and Chuan He (UChicago) - CBC Scholars “Loop Connections” Seminar (2013):
▸ Reversible RNA and DNA Methylation in Biological Regulation
Chuan He (UChicago) — Seminar Speaker - 10th Annual CBC Symposium (2012):
▸ Epigenomics
Chuan He (UChicago) — Symposium Speaker - CBC Catalyst Award (2011):
▸ Capturing Kinetically Labile Multiprotein Assemblies on DNA by Chemical Crosslinking
PIs: Jung-Hyun Min (UIC) and Chuan He (UChicago) - CBC Catalyst Award (2009):
▸ Virulence and latency regulation in M. tuberculosis
PIs: Chuan He (UChicago) and Scott Franzblau (UIC)
ARTICLES PUBLISHED IN THE PAST ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
June 4, 2019
▸ Introducing Jump-Seq
Lulu Hu, a postdoc in Chuan He’s lab at UChicago and a recipient of a CBC Postdoctoral Research Award, publishes the results of her project in Journal of American Chemical Society
May 20, 2019
▸ Nano-hmC-Seal helps identify neuroblastoma biomarkers
Four UChicago scientists with multiple ties to the CBC contribute to a recent study: Bob Grossman, Lucy Godley, Barbara Stranger and Chuan He
May 14, 2019
▸ To better understand SMA
CBC Catalyst Awardee Yongchao Ma, NU, contributes to a recent publication that explores molecular mechanisms of the spinal muscular atrophy or SMA
November 10, 2017
▸ Chuan He, University of Chicago chemist and CBC multiple-times awardee, receives the prestigious 2017 Paul Marks Prize for Cancer Research
February 17, 2016
▸ RNA Modification Discovery Suggests New Code for Control of Gene Expression
January 14, 2015
▸ Finding Damaged DNA — Needle in the Haystack
May 22, 2012
▸ Unveiling Hidden DNA Code
March 2, 2012 (updated March 16, 2012)
▸ CBC Scholars Rock!
October 24, 2011
▸ Spotlight on October 2011 Publications by Three CBC Award Research Teams
